By Victor V Rao MBBS, DMRD, RDMS
Introduction
A 39-year-old male presented to the hospital with pain in the right mid-thigh region for one week. The patient had no significant past medical history. The patient did give a history of travel to Africa a few months ago. The pain was localized to the right upper inner thigh region. A 1.5 cm tender red nodule was seen in the right mid-thigh. There was no history of trauma or insect bites. The patient was prescribed broad spectrum antibiotics and was recommended to follow up after 10 days.
The patient returned after 10 days and complained of no relief. The lesion was still tender, slightly enlarged and about 2.5 cm in diameter. A tiny opening was seen in the middle of the lesion.
The patient recollected that he thought he saw a thin white thread like structure appear momentarily from the tiny opening in the skin lesion when he was in a bathtub. He tried to grab the thread like structure, but it seemed to retract back into the lesion. The patient reported not having seen that thread like structure ever since. This was baffling to the physician. It was time to switch to POCUS mode and scan the nodule using a high frequency linear ultrasound transducer to assess the internal structure of the skin lesion. Did the patient really see something? Was there really a worm inside the skin lesion? Was that even possible? POCUS examination of the skin and soft tissue would reveal the truth as always.
A B-mode ultrasound examination was performed using a high frequency linear transducer with the soft tissue superficial preset setting. A well-defined hypoechoic lesion was seen in the subcutaneous tissue. The lesion was slightly compressible with the ultrasound transducer. There were multiple thin hyperechoic tubular structures seen within the skin lesion. On real-time B-mode ultrasound the worm like structure was seen to be slowly moving within the skin lesion. A diagnosis of subcutaneous dirofilariasis with a live worm was made within minutes. The lesion was surgically removed with the live worm in it.
Dirofilariasis is a parasitic infection caused by filarial worms of the genus Dirofilaria, most commonly infecting dogs, cats, foxes, and other wild mammals but occasionally infecting humans. The infection is spread by a bite from infected mosquitoes such as Culex, Aedes and Anopheles. Let us take a moment to briefly review the life cycles of the two types of Dirofilaria which can sometimes infect humans. See Figure 1 below.
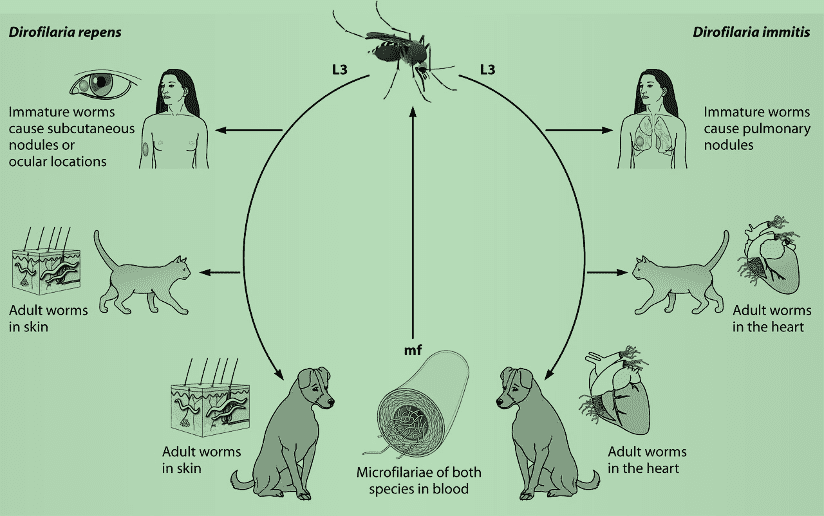
Figure 1. Life cycles of D. immitis and D. repens. mf, microfilaremia.
Ultrasound can play a significant role in the diagnosis of dirofilariasis, particularly in cases involving the lungs or subcutaneous tissues. We will briefly look at the role of ultrasound in the diagnosis of these two forms of dirofilariasis that affect humans.
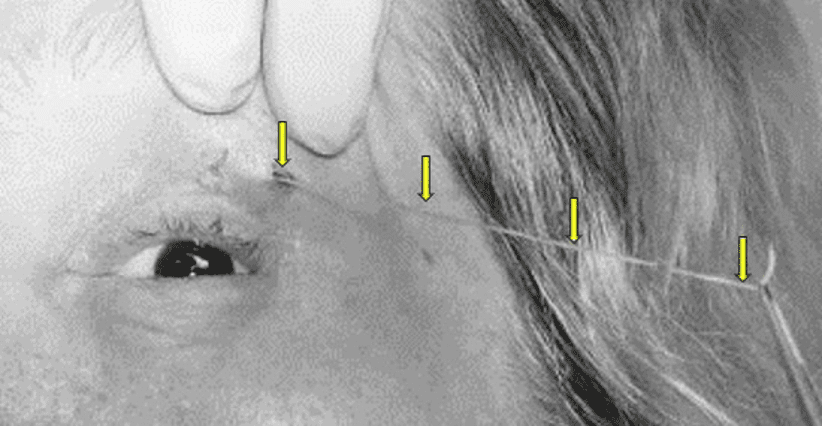
Figure 2. Subcutaneous Dirofilariasis. The worm is being extracted from the opening in the skin lesion (nodule with a small hole in the center) in a different patient. Pull very gently to avoid breaking the worm and retracting back into the nodule. The hole in the nodule may not be present in all patients.
Role of Ultrasound in Dirofilariasis Diagnosis
Subcutaneous Dirofilariasis
On ultrasound, the lesion will appear as a well-defined, hypoechoic, or heterogenous nodule in the subcutaneous tissue. The nodular lesion will be compressible with direct compression by the transducer footprint. The average size of the lesion will be 0.5 to 3.0 cm. The worm may be visualized as a structure with parallel echogenic lines with hypoechoic or anechoic center. The parallel echogenic lines with an anechoic center are like the inner tube sign on ultrasound of the roundworm Ascariasis. The worm can be seen moving within the nodule on real time ultrasound B-mode/2D mode imaging. In this case it was the Dirofilariasis repens worm. See Figure below.
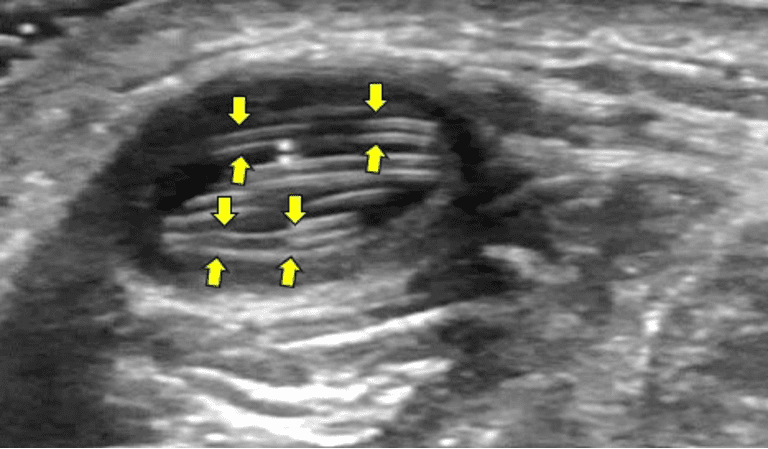
Figure 3. A well-defined oval hypoechoic lesion is seen in the subcutaneous tissue. Observe the hyperechoic parallel lines being formed by the ultrasound beam being reflected off the Dirofilaria repens worm’s near and far wall (arrows). The worm is long and thus is coiled inside the lesion to accommodate itself.
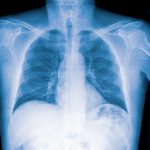
Pulmonary Dirofilariasis
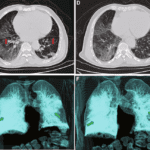
The characteristic lesion may be seen on a chest X-ray. These are caused by infection with Dirofilaria immitis. See figure below.
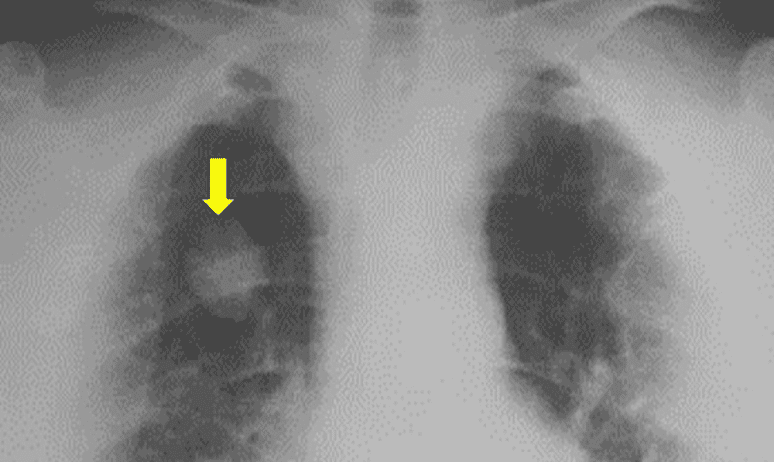
Figure 4. Chest X-ray PA view shows a single hyperdense noncalcified, pulmonary nodule (arrow) in the right upper lung field. This lesion could easily be mistaken for other pulmonary diseases such as primary lung carcinoma or metastatic lung disease.
On ultrasound examination we can see peripheral, wedge-shaped lesions in the lung. The lesion would appear as a hypoechoic or heterogeneous nodule. The lesion may appear round or oval with an average diameter of 1.0 to 3.0 cm. The lesion may show evidence of internal echoes and septations. The surrounding lung parenchyma may appear hyperechoic.
Physical Examination/Observation + Ultrasound Assisted Diagnosis
Ocular Dirofilariasis
On ultrasound examination hyperechoic linear structures may be seen within the eye. Sometimes the live worm can be seen with a direct inspection of the eye and the diagnosis is made on inspection. The live worm may be seen in the anterior chamber, vitreous, or subretinal space also. In those cases, ultrasound imaging will be helpful. Make sure to use the “low acoustic power ophthalmic ultrasound preset” to avoid high power ultrasound energy exposure to the eye which could potentially damage the eye structures.
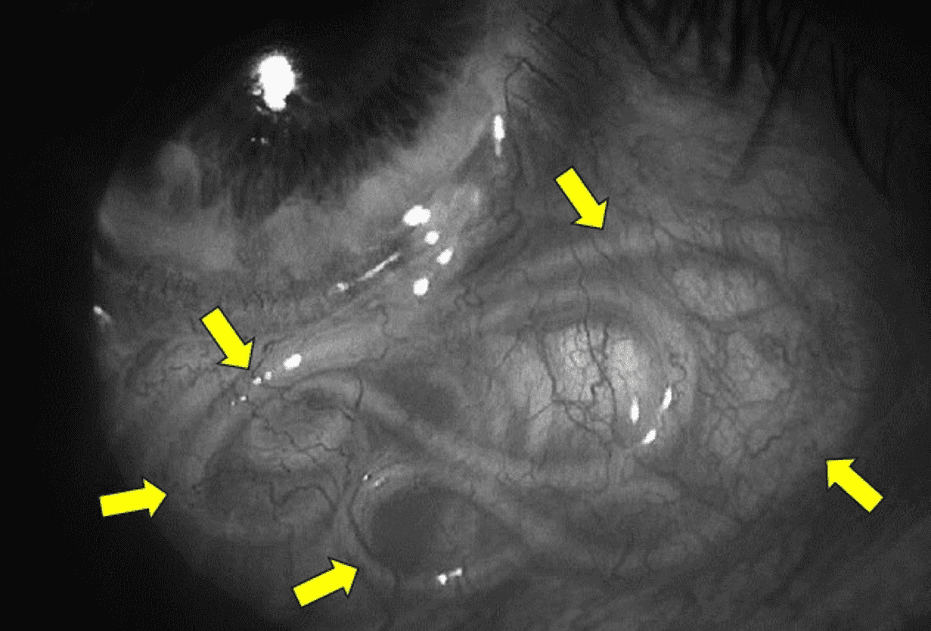
Figure 5. A white worm (arrows) is in the subconjunctival space in the patient’s eye. The worm is coiled over to accommodate itself in the space. Image courtesy of Researchgate.net
Advantages of POCUS in Diagnosis of Dirofilariasis
POCUS is a non-invasive imaging choice and does not expose the patient to unnecessary ionizing radiation. It provides real-time imaging and allows us to observe and document the movement of the worm if the worm is alive. Ultrasound may help in differentiating dirofilariasis from other serious medical conditions such as malignancies or metastatic lesions. Ultrasound guided biopsy or even CT (Computed Tomography) guided biopsy may be needed for deeper lesions. POCUS also allows easy follow up to check the progress or reappearance of lesions.
Limitations of POCUS
Ultrasound findings in lung lesions could be non-specific. Always correlate with the patient’s clinical history and physical examination findings. Always use more tests to confirm the diagnosis. Do confirm with serology and other laboratory tests. Be aware that deep lesions may not be adequately visualized with ultrasound. Operator dependence can make a difference in image acquisition and interpretation. Fortunately, the skin nodule lesion ultrasound image is quite characteristic and could lead to a spot diagnosis in seconds.
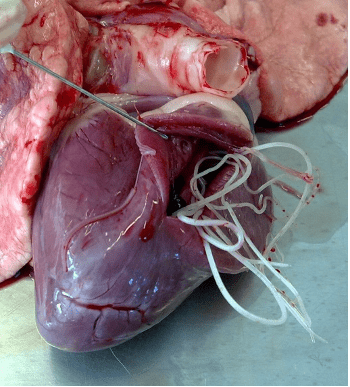
Figure 6. Male and female adult worms of D. immitis in the heart of a dog.
Final Thoughts
Even though dirofilariasis in humans is a rare condition, it is advisable for clinicians around the world to be aware of this condition to avoid the possibility of incorrect diagnosis, unnecessary antibiotic treatment, and mismanagement. If a patient presents with a migrating skin lesion this diagnosis should be on the top of your differential diagnosis list until ruled out. Soft tissue ultrasound examination of the skin lesion with a high frequency linear ultrasound transducer would be the ideal next step.
Once diagnosed, the lesion should be surgically removed with the dirofilariasis worm inside it. The role of ivermectin in patients with a skin lesion is not clearly defined. It may prevent the migration of the parasite to other areas. If secondary lesions such as in the lung and abdomen are suspected, a course of ivermectin and diethylcarbamazine is recommended. If the patient is suspected to have cardiopulmonary dirofilariasis, treatment with tetracycline is recommended. To eliminate adult worms and to reduce the risk of pulmonary embolism (PE), long term doxycycline and ivermectin are recommended as well.
One patient was reported to even undergo a mastectomy for possible suspected breast cancer which turned out to be breast tissue dirofilariasis. This scenario could have been easily avoided by examining the lesion with ultrasound. Refer the patient to an infectious disease specialist, when possible, for proper management. Please note that the treatment options described above are general guidelines and full treatment protocol is beyond the scope of this blog. The goal of this blog is to increase awareness of this condition and help make a fast and accurate diagnosis of Dirofila with POCUS followed by proper and prompt management.
References